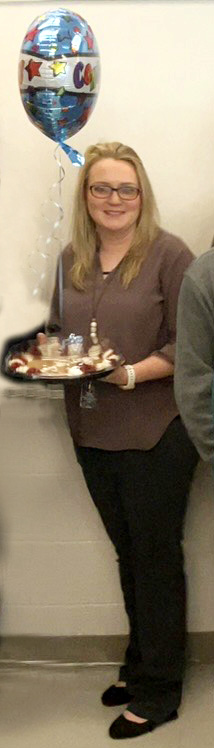Morristown West
Student & Faculty awards
March 2024 Students of the Month

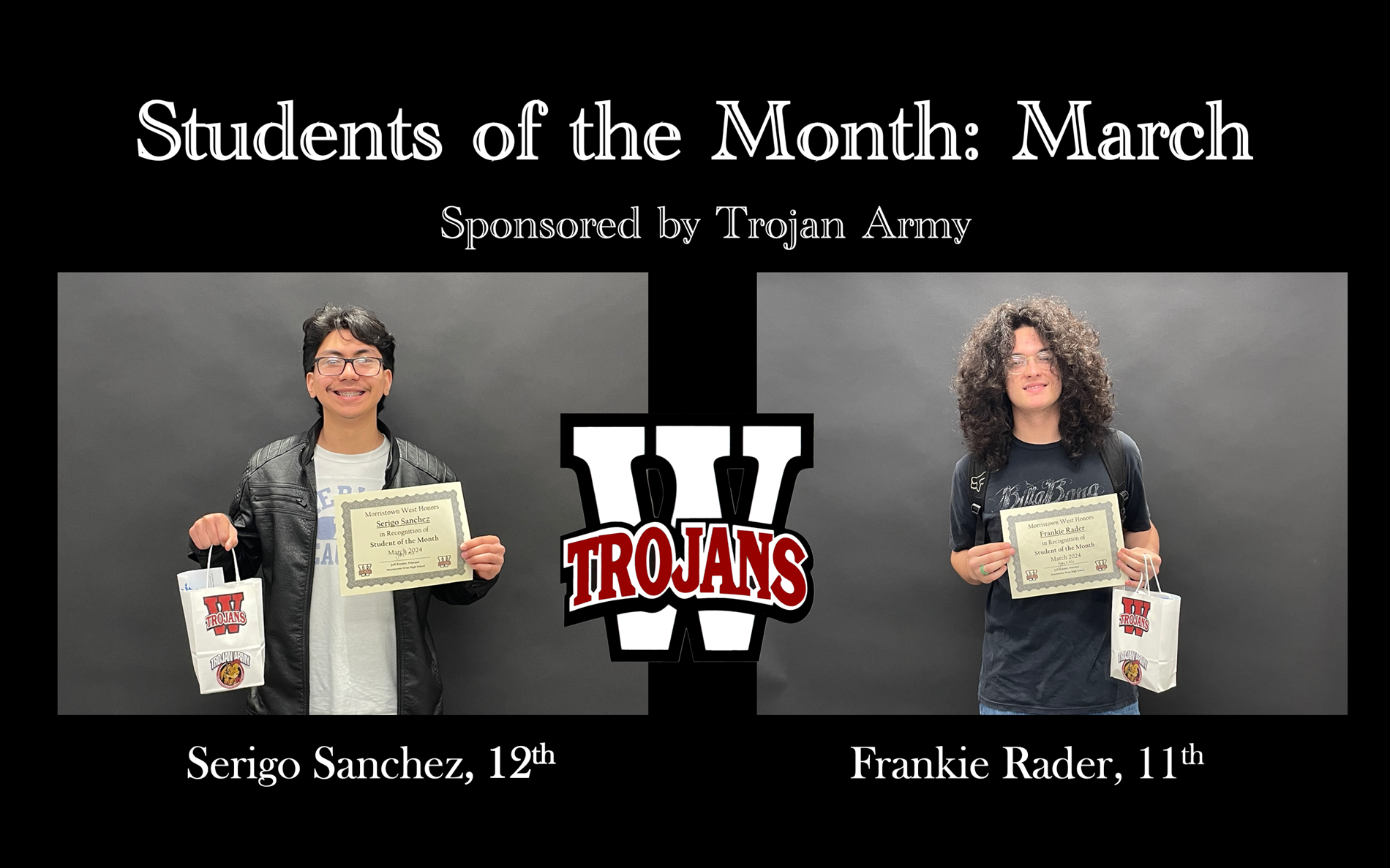
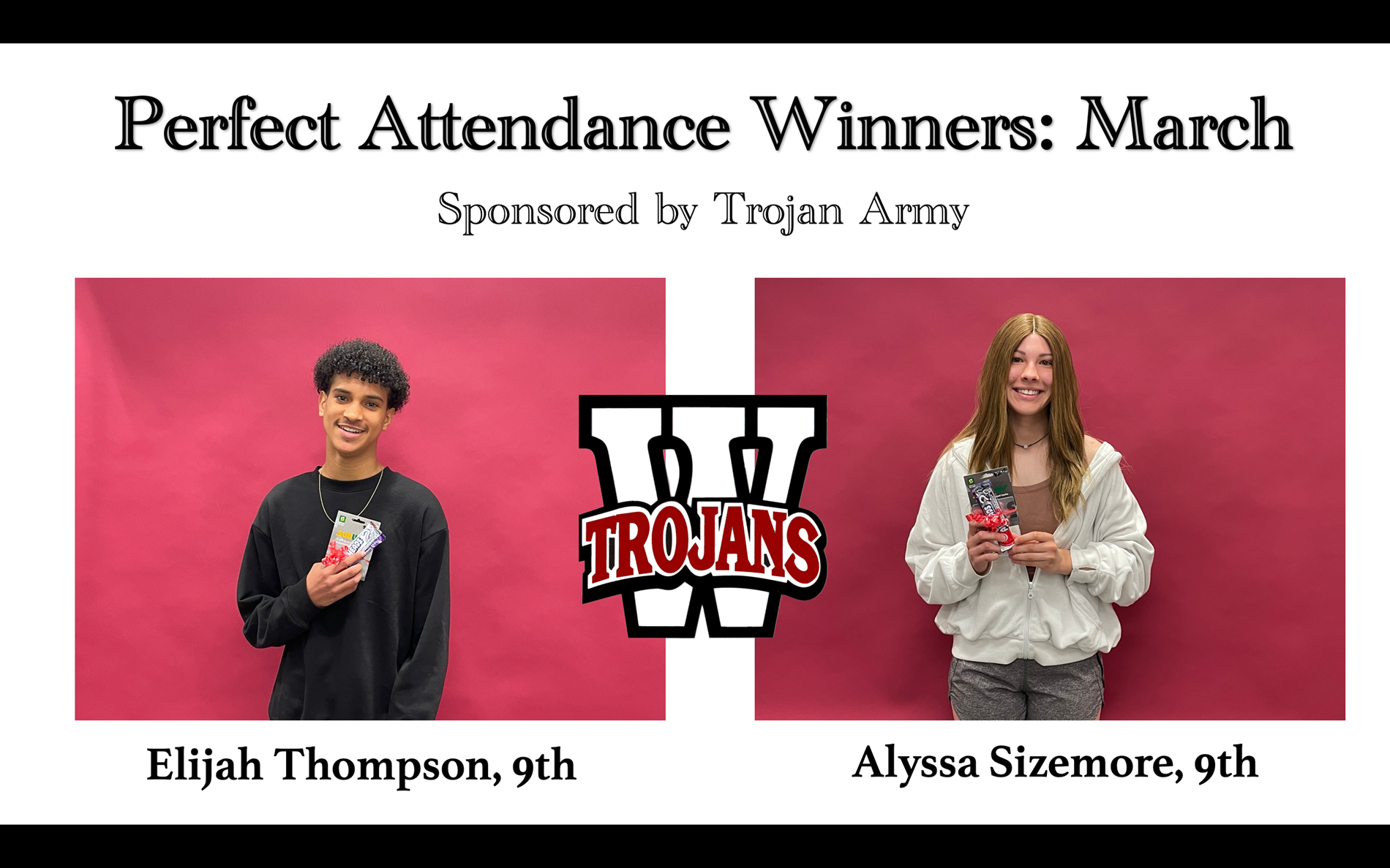
Sponsored by the Trojan Army
Sponsored by Lowland Credit Union

March perfect attendance
Congratulations Ms. Tara Phillips!
Hamblen County District High School Teacher of the Year